Abstract
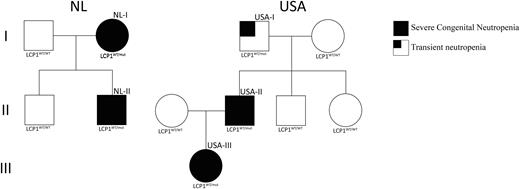
The spectrum of genetic abnormalities causing immune deficiencies is still far from complete. We identified independent kindreds in the Netherlands (NL) and the USA in which affected members presented with severe chronic neutropenia (SCN), but lacked the thus far known mutations causing SCN. Bone marrow morphology of the patients showed large hyper-segmented and binucleate neutrophils. In addition to neutropenia, lymphopenia and abnormalities in B cell development were seen, indicating that the hematopoietic defect was not restricted to the neutrophil lineage. An additional remarkable and thus far little-known feature was the presence of cells in the bone marrow with a tetraploid karyotype (Hochberg et al., Pediatr Blood Cancer 2008). Pedigree analysis of the NL and USA families revealed a hereditary pattern consistent with an autosomal dominant inheritance (Figure 1). Comparative whole exome sequencing of both pedigrees identified LCP1, encoding the actin-bundling protein L-plastin, as the single candidate gene, completely segregating affected from unaffected individuals.
The LCP1 mutations in the pedigrees are not identical, but give rise to the same altered transcript. The LCPNLmutation is a single nucleotide substitution that disrupts the splice acceptor site at the 5' side of exon 8. In contrast, the mutation LCP1USA causes a deletion of 25 nucleotides at the boundary of intron 7 and exon 8. Theoretically, the LCP1USAmutation could cause a frameshift deletion in exon 8 resulting in a premature stop codon in exon 8. However, because this mutation also disrupts the 5' splice acceptor site of exon 8, it could also lead to alternative RNA splicing, using an alternate cryptic splice acceptor site in exon 8. Sanger sequencing on cDNA derived from mRNA from NL-I unveiled that the splice-site mutation indeed results in an alternative splicing variant with an in-frame loss of 24 nucleotides at the start of exon 8. Next generation RNA sequencing on bone marrow cells from NL-I and USA-III (Figure 1) revealed that the identical alternative splice variant is expressed from LCP1USA and LCP1NL. In both pedigrees, there is approximately a fifty percent drop in reading depth from the start of exon 8 up until the next available splice acceptor site, consistent with a heterozygous 24-nucleotide deletion from the start of exon 8. The resulting in-frame deletion of 8 amino acids affects a highly conserved region between actin binding domain (ABD) 1 and ABD2, thereby predicted to affect the horseshoe-like 3D structure of L-plastin. LCP1USA and LCP1NL will henceforth collectively be termed LCP1ex8mut.
Confocal microscopy analysis of HeLa cells expressing LCP1ex8mut showed that mutant L-plastin colocalized with F-actin in excessively formed filopodia, which was not seen in cells expressing wild-type LCP1 (LCP1wt). In addition, binucleate cells were present in cells expressing LCP1ex8mut, but not LCP1wt. A number of cells in G2M phase showed accumulation of F-actin and L-plastin on the contractile ring, a characteristic seen only in cells expressing LCP1ex8mut. This finding suggests that mutant L-plastin interferes with successful completion of cell division. Accumulation of mutated LCP1 on the contractile ring thus could explain the inability of neutrophil progenitors to properly go through cytokinesis, hence giving rise to neutropenia and tetraploidy.
Finally, because a recently developed mouse model revealed that phosphorylation of serine 5 (Ser5) of L-plastin is essential for its function in host-pathogen interactions and immune defense in vivo (Anaya et al., J Immunol 2021), we mutated Ser5 to alanine (Ser5Ala) in LCP1ex8mut. This did not affect the function of LCP1ex8mut, suggesting that the mutation confers activation of L-plastin independent of upstream serine/threonine kinase pathways. Altogether, these findings establish that the mutations confer a gain of function rather than haploinsufficiency.
In conclusion, by comparing independent pedigrees, we have identified a new immune deficiency syndrome caused by autosomal dominant activating mutations in LCP1 leading to defective neutrophil production and B cell development, which at least in part results from disturbed cytokinesis causing tetraploidy.
Figure1. Pedigree information of the NL and USA family. Solid black squares or circles indicate neutropenic males or females, respectively.
Disclosures
Newburger:UpToDate: Patents & Royalties: Section Editor; X4 Pharmaceuticals, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal